CEOs of NorDx Laboratories, Sonora Quest Laboratories, and HealthPartners/Park Nicollet Laboratories expect demand for SARS-CoV-2 tests to only increase in coming months
AUSTIN, TEXAS—For clinical laboratories and anatomic pathology groups in the United States, the single most urgent question is this: how long will the need for substantial volumes of COVID-19 testing continue? Last Wednesday, that question was answered in a most definitive way by CEOs of three nationally-prominent clinical laboratory organizations during a general session of the virtual Executive War College on Clinical Laboratory and Pathology Management.
The short answer is that large volumes of COVID-19 testing will be needed for the remaining weeks of 2020 and substantial COVID-19 testing will occur throughout 2021 and even into 2022. This has major implications for all clinical laboratories in the United States as they plan budgets for 2021 and attempt to manage their supply chain in coming weeks. The additional challenge in coming months is the surge in respiratory virus testing that is typical of an average influenza season.
The title of this information-filled general session was “Coming Next to Clinical Laboratory and Pathology: A Robust Panel Discussion of How Labs Can Prosper Clinically and Financially Going Forward.” Chair and Moderator for the panel was Robert L. Michel, Publisher and Editor-in-Chief of The Dark Report and Dark Daily.

The panelists were:
- David Dexter (above left), President and CEO of Sonora Quest Laboratories, LLC, a joint venture between Quest Diagnostics (NYSE:DGS) and Banner Health in Tempe, Arizona. He also serves as President and CEO for Laboratory Sciences of Arizona, LLC.
- Stan Schofield (above center), President of NorDx, a regional laboratory corporation that supports an integrated delivery system at MaineHealth in Portland, Maine.
- Rick L. Panning (above right), MBA, MLS(ASCP)CM, retired as of Oct. 2 from the position of Senior Administrative Director of Laboratory Services for HealthPartners and Park Nicollet in Minneapolis-St. Paul, Minnesota.
Each panelist was asked how his parent health system and clinical laboratory was preparing to respond to the COVID-19 pandemic through the end of 2020 and into 2021.
First to answer was Panning, whose laboratory serves the Minneapolis-Saint Paul market.
A distinguishing feature of healthcare in the Twin Cities is that it is at the forefront of operational and clinical integration. Competition among health networks is intense and consumer-focused services are essential if a hospital or physician office is to retain its patients and expand market share.
Panning first explained how the pandemic is intensifying in Minnesota. “Our state has been on a two-week path of rising COVID-19 case numbers,” he said. “That rise is mirrored by increased hospitalizations for COVID-19 and ICU bed utilization is going up dramatically. The number of hospitalized COVID-19 patients has doubled during this time and Minnesota is surrounded by states that are even in worse shape than us.”
These trends are matched by the outpatient/outreach experience. “We are also seeing more patients use virtual visits to our clinics, compared to recent months,” noted Panning. “About 35% of clinical visits are virtual because people do not want to physically go into a clinic or doctor’s office.
“Given these recent developments, we’ve had to expand our network of specimen collection sites because of social distancing requirements,” explained Panning. “Each patient collection requires more space, along with more time to clean and sterilize that space before it can be used for the next patient. Our lab and our parent health system are focused on what we call crisis standards of care.
“For all these reasons, our planning points to an ongoing demand for COVID-19 testing,” he added. “Influenza season is arriving, and the pandemic is accelerating. Given that evidence, and the guidance from state and federal officials, we expect our clinical laboratory will be providing significant numbers of COVID-19 tests for the balance of this year and probably far into 2021.”
COVID-19 Vaccine Could Increase Antibody and Rapid Molecular Testing
Arizona is seeing comparable increases in new daily COVID-19 cases. “There’s been a strong uptick that coincides with the governor’s decision to loosen restrictions that allowed bars and exercise clubs to open,” stated Dexter. “We’ve gone from a 3.8% positivity rate up to 7% as of last night. By the end of this week, we could be a 10% positivity rate.”
Looking at the balance of 2020 and into 2021, Dexter said, “Our lab is in the midst of budget planning. We are budgeting to support an increase in COVID-19 PCR testing in both November and December. Arizona state officials believe that COVID-19 cases will peak at the end of January and we’ll start seeing the downside in February of 2021.”
The possible availability of a SARS-CoV-2 vaccine is another factor in planning at Dexter’s clinical laboratory. “If such a vaccine becomes available, we think there will be a significant increase in antibody testing, probably starting in second quarter and continuing for the balance of 2021. There will also be a need for rapid COVID-19 molecular tests. Today, such tests are simply unavailable. Because of supply chain difficulties, we predict that they won’t be available in sufficient quantities until probably late 2021.”
COVID-19 Testing Supply Shortages Predicted as Demand Increases
At NorDx Laboratories in Portland, Maine, the expectation is that the COVID-19 pandemic will continue even into 2022. “Our team believes that people will be wearing masks for 18 more months and that COVID-19 testing with influenza is going to be the big demand this winter,” observed Schofield. “The demand for both COVID-19 and influenza testing will press all of us up against the wall because there are not enough reagents, plastics, and plates to handle the demand that we see building even now.
“Our hospitals are already preparing for a second surge of COVID-19 cases,” he said.
COVID-19 patients will be concentrated in only three or four hospitals. The other hospitals will handle routine work. Administration does not want to have COVID-19 patients spread out over 12 or 14 hospitals, as happened last March and April.
“Administration of the health system and our clinical laboratory think that the COVID-19 test volume and demand for these tests will be tough on our lab for another 12 months. This will be particularly true for COVID-19 molecular tests.”
As described above, the CEOs of these three major clinical laboratories believe that the demand for COVID-19 testing will continue well into 2021, and possibly also into 2022. A recording of the full session was captured by the virtual Executive War College and, as a public service to the medical laboratory and pathology profession, access to this recording will be provided to any lab professional who contacts info@darkreport.com and provides their email address, name, title, and organization.
Executive War College Closing Session
This week’s closing general session of the virtual Executive War College also will deal with the current state of the clinical laboratory industry and bring together three notable lab industry leaders and thinkers. The session, titled “What Comes Next in Healthcare and Laboratory Medicine: Essential Insights to Position Your Clinical Lab and Pathology Group for Clinical and Financial Success, Whether COVID or No COVID,” takes place Thursday, Oct. 29, 2020, from 2:00 PM to 3:00 PM Eastern.
Your presenters will be:
- John Kershaw, Chair and Moderator—President and Chief Executive Officer, Sysmex America, Lincolnshire, Illinois.
- Bob McGonnagle, Panelist—Publisher, CAP TODAY, Northfield, Illinois.
- Robert L. Michel, Panelist—Publisher, Editor-in-Chief, The Dark Report and Dark Daily, Spicewood, Texas.
Given the importance of sound strategic planning for all clinical laboratories and pathology groups during their fall budget process, the virtual Executive War College is opening this session to all professionals in laboratory medicine, in vitro diagnostics, and lab informatics.
To register for access, visit: https://dark.regfox.com/executive-war-college-2020. Enter the code: COURTESYCAPTDR. Next, select “apply code” and complete the registration.
—Michael McBride
Related Information:


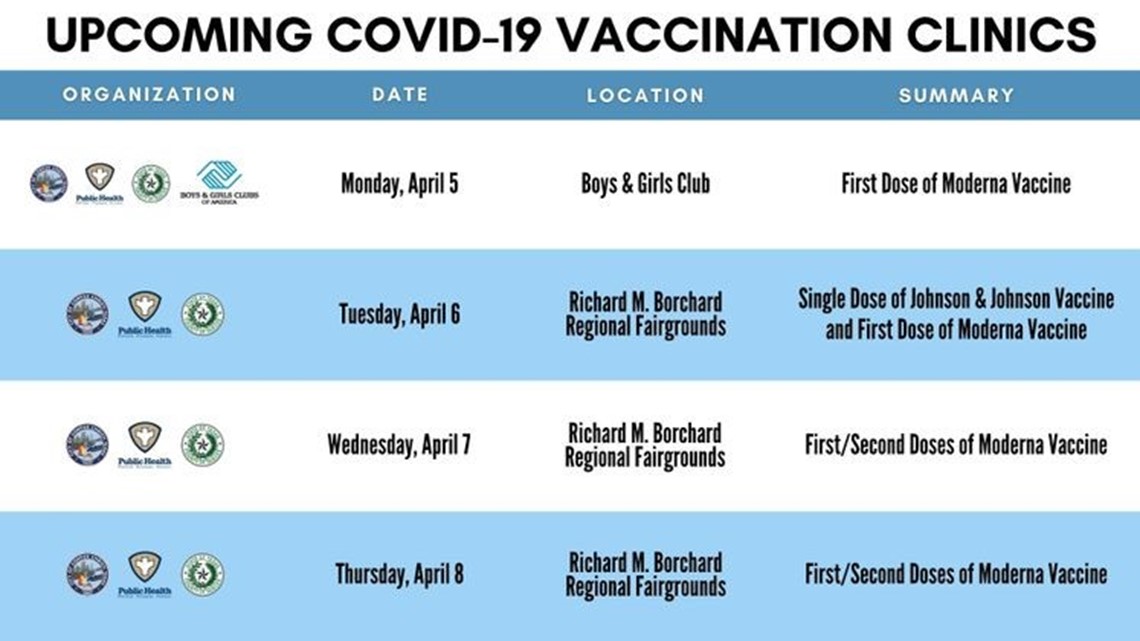
 Something went wrong.More VideosNext Up40 years later, a survivor speaks out about the Grain Elevator accident on the Corpus Christi Ship CLanes on Harbor Bridge will close for safety inspections this weekVolume 90% Author: James Ayala, Haley WilliamsPublished: 3:12 PM CST January 15, 2021Updated: 7:28 AM CDT April 8, 2021
Something went wrong.More VideosNext Up40 years later, a survivor speaks out about the Grain Elevator accident on the Corpus Christi Ship CLanes on Harbor Bridge will close for safety inspections this weekVolume 90% Author: James Ayala, Haley WilliamsPublished: 3:12 PM CST January 15, 2021Updated: 7:28 AM CDT April 8, 2021